drug carrier
Application of PLGA nanospheres for skincare cosmetics
Hiroyuki Tsujimoto, Hosokawa Micron Corporation
CONTENT
1. Introduction2. Safety and applicability of PLGA nanospheres for skincare cosmetics
2-1 Safety of PLGA nanospheres
2-2 Stability of PLGA nanospheres
3. PLGA nanospheres functionality and application for skincare technology3-1 Skin permeability of PLGA nanospheres
3.1.1 Evaluation of PLGA nanospheres in terms of permeability and
drug deliverability
3.1.2 Evaluation of PLGA nanospheres in terms of permeability (with
mouse / horizontal altered cell)
3.1.3 Evaluation of drug solution in terms of skin permeability (with human
skin tissue model / Franz diffusion cell)
3-2 PLGA nanosphere composite for cosmetics
3-3 Skin and scalp care technology with PLGA nanospheres
3.3.1 Skin care technology
3.3.2 Example of application for sensitive skin care
3.3.3 Scalp care technology
Abstract
Keywords: skin whitening, skin moistening, antiaging, sensitive skin protection, hair growth, scalp care, etc.
1.Introduction
Drug Delivery Systems (DDS) are widely applicable pharmaceutical methodologies that provide three main functions: (1) delivering a drug to a specific region of the human body where requires drug administration (targeting); (2) controlling the amount and/or speed of drug release to maximize drug efficacy (controlling release); and (3) letting the region absorbing a drug consistently and safely (improving absorption). The recent active development of functional cosmetics for effective skincare is based on DDS concept, in which a skin-beautifying component refined in nano-sized particles or enclosed in such nano-sized base (carrier) as liposomes or nanospheres is passed from horny layer barrier to targeted deeper parts of skin where the effective component sustains its efficacy. The component of such base (carrier) to be applied on skin must be selected in order not only to accelerate skin permeability but also to ensure safety for skin physiology through constant application. Among potential components, Poly-lactic-co-glycolic acid (PLGA) is the newest and most suitable nanospheres to meet there requirements. This report is provided to introduce PLGA with its biocompatibility, bioabsorbability and functionality including examples of formulation in beauty essence, hair growth tonic, etc.
2. Safety and applicability of PLGA nanospheres for skincare cosmetics
2.1 Safety of PLGA nanospheres
Safety of PLGA has been verified by the following test parameters conformed to GLP in which PLGA offered all negative results: (1) toxicity by single administration (rat LD50 > 2,000 mg/kg); (2) primary skin irritation test (rabbit); (3) constant skin irritation test (rabbit); (4) sensitization test (guinea pig); (5) phototoxicity (guinea pig); (6) eye irritation test (rabbit); (7) mutagenicity test; (8) human patch test; and (9) photo sensitization test (guinea pig) (PLGA offered no ultraviolet ray absorption). PLGA is intended to be registered as an additive for quasi drug by the year 2007. PLGA is hydrolyzed in the skin to lactic acid and glycolic acid, and finally to water and carbon dioxide to be egested. Unlike another nanosphere which may questionably stay in the body, PLGA is an extremely safe base without body residue. Besides, safety of PLGA has been also confirmed through evaluating its effect to basement membrane, malignant growth of melanocytes (melanoma) and metastasis under constant application to skin as a cosmetic foundation, in which PLGA offered no effect to these parameters1) 2).
2.2 Stability of PLGA nanospheres
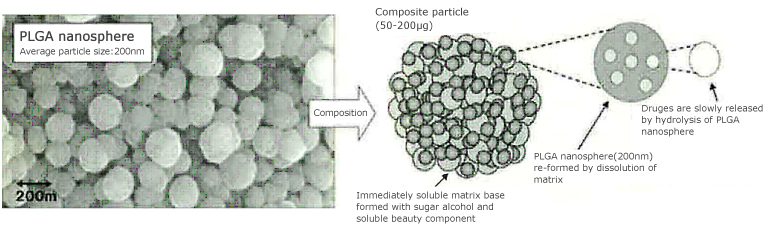
It is true that PLGA was difficult to be used practically as a cosmetic bulk because it was poor in preservation stability produced by its hydrolytic property and lower glass-transition temperature of 45°C in which particles might be coagulated by heat. However, we newly developed practically-usable drug (effective component) carrier particles to improve physical properties connecting with preservation stability, by forming structure-regulated composite particles (with dozens of micrometers in apparent size) including dispersion phase of PLGA nanospheres with 200 nm average diameter that enclose skin-beautifying component and matrix of vehicle and surface modifier, etc. (diagram 1). For instance, by mixing these composite particles with lotion or serum when using, matrix base is immediately dissolved so that nanospheres are re-formed to perform its inherent function. Diagram 1 shows particle distribution and photograph when PLGA nanospheres are re-formed immediately after the composite particles were dispersed in water.
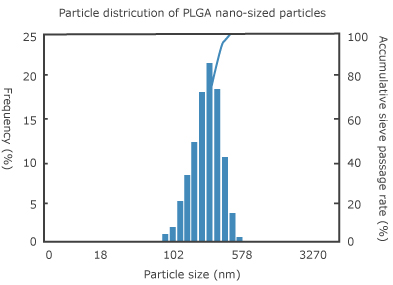 |
Diagram 1. | Particle distribution and photograph by atomic force microscopic of PLGA nanospheres dispersing in water, and schematic structure of the composite particles |