drug carrier
Once-a-Month Injectable Microcapsules of Leuprorelin Acetate
Hajime TOGUCHI, Yasuaki OGAWA, Hiroaki OKADA, *
and Masaki YAMAMOTO
Pharmaceutics Research Laboratories, Research and Development
Division, Takeda Chemical Industries, Ltd.,
Yodogawa-ku, Osaka 532, Japan
CONTENT
1. Introduction2. In vivo biodegradable polymer
3. Preparation of microcapsules
4. Drug slow-release potentiality
5. Drug concentration in serum
6. Pathological effect
7. Study for industrialization
8. Postscript
9. Acknowledgement
Abstract
Keywords – leuprorelin (leuprolide); injectable microcapsules; one-month release; copoly (lactic/glycolic acid); chemical castration; endometriosis; dosage design
1.Introduction
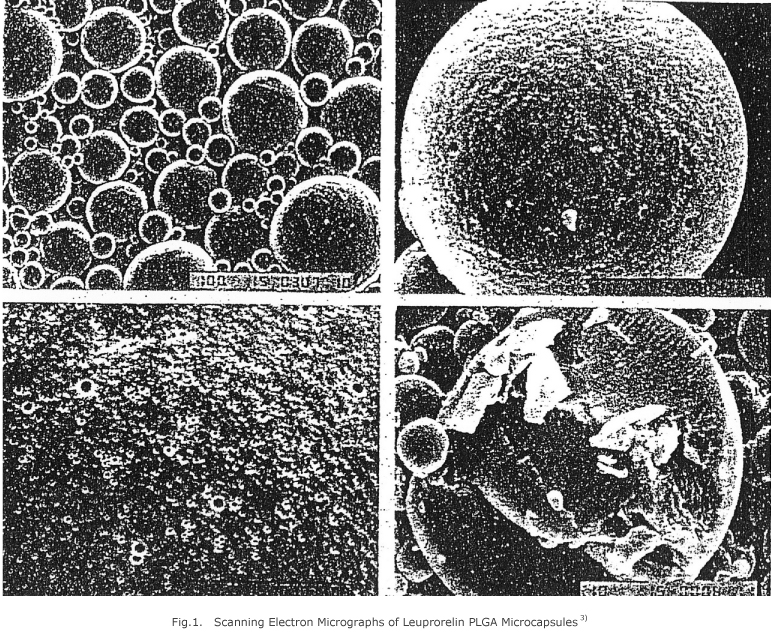
This drug formulation, being injected once a month so that the agonist can be released from administration site continuously for over a month, constitutes a new drug delivery system (DDS)1-7) effective in treating such hormone-dependent diseases as prostatic cancer, endometriosis, fibroid and so on. It is formed by injectable polycore microcapsules (MCP) of a biodegradable polymer, copolymer of DL-lactic acid and glycolic acid (PLGA) as wall material with around 20 µm in average particle size, which can be administered intramuscularly or subcutaneously by using a small needle with 22 to 23 gauge, as with an ordinary suspension agent (Fig. 1). The agonist is released slowly in accordance with speed of in vivo hydrolysis of PLGA, and it is unnecessary to remove the polymer since it disappears naturally from administration site. Dosage of this MCP to a cancer patient is approximately 100 mg in which only 7.5 mg of the agonist is contained. Unlike administration of an existing injectable solution where patients had forced themselves to conduct subcutaneous injection every day, this MCP might set patients free from pain by frequent injections and enhance administration compliance. Besides, constant and prolonged release of the agonist always stimulates target organ and enhances efficacy with more stable down regulation in which the dosage might be reduced by 1/4 to 1/8 of an existing injectable solution.
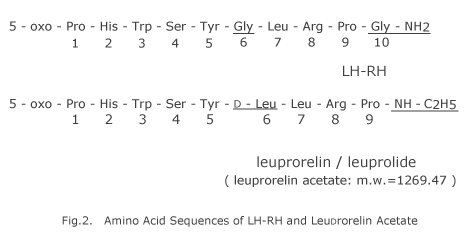
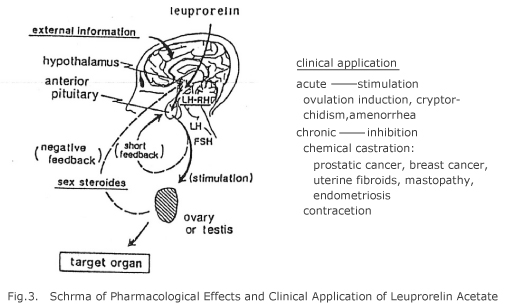
Leuprorelin (leuprolide, D-Leu6-(des-Gly10-NH2)-LH-RH ethylamide acetate) is a new super-active agonist of luteinizing hormone-releasing hormone (LH-RH) synthesized by our Dr. Fujino and his group for the first time in the world, which may offer activation nearly 100 times stronger than LH-RH (Fig. 2). Normally, this compound acts on pituitary gland in the brain by which luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are released, and in consequence, activation of testis or ovary takes place that enhances biosynthesis and secretion of sexual steroid (Fig. 3). However, frequent injection of high-dose LH-RH leads to down regulation of LH-RH receptor of pituitary gland and inversely inhibits biosynthesis of sexual steroid in which therapeutic antagonistic activity takes place to bring genital atrophy. This high-order gonadal inhibition via pituitary gland is equivalent to surgical castration, that is, so-called “chemical castration” might be produced. In recent years, by utilizing such function with similar hyperactive LH-RH agonist, hormone-dependent diseases such as prostatic cancer,10,11) breast cancer,12,13) endometriosis,14,15) fibroid,16,17) childhood central precocious puberty13) and the like could be treated without surgical operation (Fig. 3).
 Meanwhile, these therapeutic effects could be realized and maintained by prolonged administration of these compounds of highly aqueous soluble peptide containing the sequence of 9 - 10 amino acids. They have, however, low mucosal permeability and high rate of in vivo disappearance, so that prolonged and frequent injection is inevitable in most cases. In order to overcome this point, we had made study of developing a simple and stable DDS from the outset. Initially, we had launched in studying a formulation for mucosal self-administration which hardly create pain. Since it was mainly targeted to treat breast cancer, specific study was made with comparatively well-absorbable mucosa namely vagina and nose, in which an interesting sorbefacient was detected with its potential. As the result of initial clinical trial, however, we had finally given up its practical use because it offered inferior absorbency to the one detected by preliminary animal test and its unevenness depending on physiological factor. One fruit through study of this formulation was that stronger antagonistic therapeutic effect could be enhanced by maintaining the level of agonist in the blood and keeping stimulating receptor. As to another clinical trial for breast cancer conducted with an injectable solution, we couldn’t obtain a high initial cure rate as we expected so that we re-focused target to
Meanwhile, these therapeutic effects could be realized and maintained by prolonged administration of these compounds of highly aqueous soluble peptide containing the sequence of 9 - 10 amino acids. They have, however, low mucosal permeability and high rate of in vivo disappearance, so that prolonged and frequent injection is inevitable in most cases. In order to overcome this point, we had made study of developing a simple and stable DDS from the outset. Initially, we had launched in studying a formulation for mucosal self-administration which hardly create pain. Since it was mainly targeted to treat breast cancer, specific study was made with comparatively well-absorbable mucosa namely vagina and nose, in which an interesting sorbefacient was detected with its potential. As the result of initial clinical trial, however, we had finally given up its practical use because it offered inferior absorbency to the one detected by preliminary animal test and its unevenness depending on physiological factor. One fruit through study of this formulation was that stronger antagonistic therapeutic effect could be enhanced by maintaining the level of agonist in the blood and keeping stimulating receptor. As to another clinical trial for breast cancer conducted with an injectable solution, we couldn’t obtain a high initial cure rate as we expected so that we re-focused target to prostatic cancer, more hormone dependent (70 – 80 %).
Prostatic cancer cases have been largely detected in Europe and USA, specifically 80,000 patients have been newly detected annually in USA, and it follows lung cancer in mortality rate by cancer. Besides, prostatic cancer is relatively slow in its development, but it is so bone-crushing that in most cases the cancer has already metastasized to bone when detected. As to significant properties of this agonist, it is enough effective against metastatic cancer in the event that the original cancer is hormone dependent, namely it offers equivalent efficacy to surgical castration without metal and physical pain. And unlike existing estrogenic therapy which may produce severe side effects such as myocardial infarction and thrombosis, this DDS never give such side effects. 28)
Accordingly, we had intently changed the direction of our pharmaceutical study, in which we launched in studying and developing a prolonged slow-release drug formulation for the purpose of minimizing the number of dosing and realizing more stable therapeutic effect. As a result, we made success in putting once-a-month injectable microcapsules to practical use 29-32) through the use of lactic/glycolic acid, biodegradable polymer and novel in-water drying method established by us. Simultaneously with our success, ICI, Southern Research and Syntex had been pursuing the development of implantable agents based on LH-RH derivative of PLGA and/or injectable microcapsules by applying phase separation method. Despite the development of such counterparts, we first acquired manufacture license in USA in January 1989. This suggests some advantages of our product in terms of easy administration (local anesthesia-free, the use of a small needle, etc.), constant drug release, little residual solvent, avoidance of radiation sterilization by complete sterile environment and technique, etc.
In this article, we review the details of research and development of slow-release injectable formulation.
2.In vivo biodegradable polymer
In initiating the development of prolonged slow-release injectable formulation, we studied release control by using microcapsules with wall compound made of poorly aqueous-soluble polymer. Since the polymer must disappear from injection site, we decided to choose the one in vivo biodegradable, and focused on polylactic acid (PLA) or PLGA (Fig. 5: random copolymer of DL-lactic acid and glycolic acid is used in this formulation) which is already proven in practical use for a long time. We determined in vivo biodegradability by synthesizing more than a dozen sorts of polymers and implanting them subcutaneously in rats. 38) These polymers have superior in vivo affinity 39) and the highest level of safety, hydrolyzing within an organism to in vivo egestive substances including lactic acid, glycolic acid, carbon dioxide and water.
 |
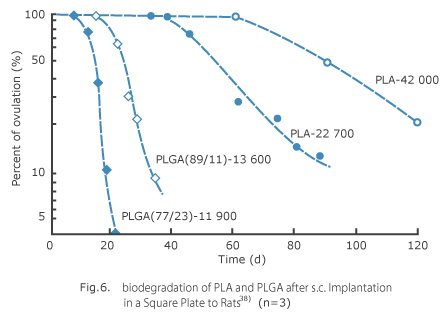 |
For synthesizing low-molecular-weight polymer used for determination of in vivo biodegradability, polymerization method via direct condensation invented by Nevin and his team 40) was adopted in which monomers of DL-lactic acid and glycolic acid are polymerized with strong acid ion-exchange resin catalyst. And high-molecular-weight polymers were synthesized according to ring-opening polymerization method invented by Woodland and his team 41) in which dimmer (DL-lactide) is polymerized with diethyl zinc as a catalyst. These polymers were evaluated in terms of polymerization ratio between lactic and glycolic acid by nuclear magnetic resonance spectrum (NMR), and molecular weight by gel permeation chromatography (GPC). Additionally, by using these polymers, square plates with 1-mm thickness were made by melting and compression method, cut into 10 x 10 mm, implanted subcutaneously in rats, taken out at specified intervals, and their change of weight was determined. As an example of such change shown in Fig. 6, lag time without any change of weight was seen at an initial stage, but later weight was reduced along with sigmoid curve. As approximating such curve by first-order kinetics and regarding time span during which 100 wt % remained as lag time, rate of elimination was measured through its slope and half life was calculated. As to rate of elimination of polymer, the higher molecular weight, the longer lag time and half life lengthened proportionally (Fig. 7), and as to PLGA, the higher copolymer ratio of glycolic acid by 50 %, the faster the PLGA was biodegraded and eliminated (Fig. 8).
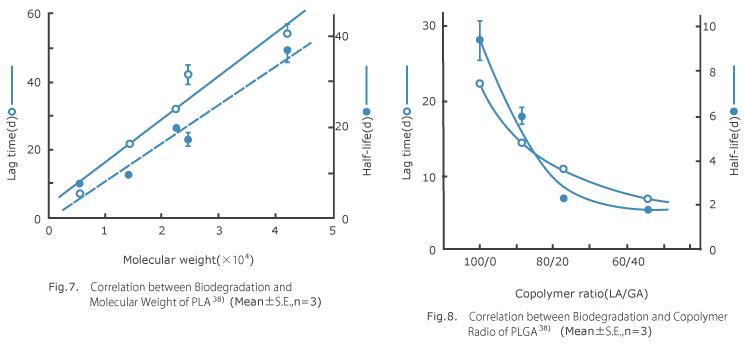
Polymer in vivo elimination patterns might be presumed as follows. 7) By in vivo administration, first water molecules quickly penetrate into polymer in which polymer is hydrated and swelled, and its molecular weight is gradually reduced along with progress of hydrolysis to the whole polymer. Then, simultaneously with generation of water-soluble oligomer, polymer weight begins to be reduced accordingly. Duration of these processes is equivalent to the afore-mentioned lag time. After that, weight was gradually reduced at an approximate rate of first-order kinetics along with the rate of hydrolysis. Meanwhile, as to base material of once-a-month slow-release drug formulation, disappearing from administration site within 1 to 2 months is preferable. Fig. 6 might suggest that such a polymer with a comparatively low molecular weight could meet the purpose as PLGA (77/23)-11900 or PLGA (89/11)-13600 (lactic acid/glycolic acid copolymer ratio – weight-average molecular weight) offering 1 or 2-week lag time and about 7-day half life. Namely, appropriate polymer should have a molecular weight of several thousands in case of PLA, or ten thousands and several thousands in case of PLGA with copolymer ratio between 90/10 and 50/50. However, we concluded that PLGA (75/25) was the most suitable, in consideration of facts that PLGA comprising 50 % or more glycolic acid in copolymer ratio becomes much inferior in solubility with organic solvent which is difficult to be prepared by after-mentioned in-water drying method, and little change of biodegradation rate is produced depending on copolymer ratio (section of gentle curve shown in Fig. 8).
Besides, we found through subsequent evaluation of releasability of MCP prepared with various polymers that, if it contained more water-soluble oligomer, polymer became unstable which led to increase of initial release, so-called “tunnel effect”. This obviously reveals that it is necessary to remove oligomer as much as possible and control molecular weight distribution. 42) Since later we could foresee formulation art, in collaboration with Wako Pure Chemical Industries, we developed synthesis system being free of residual condensation catalyst by which polymer could be mass-produced. 43)