drug carrier
Guide Line
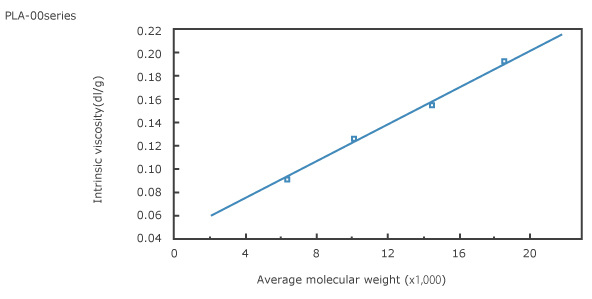
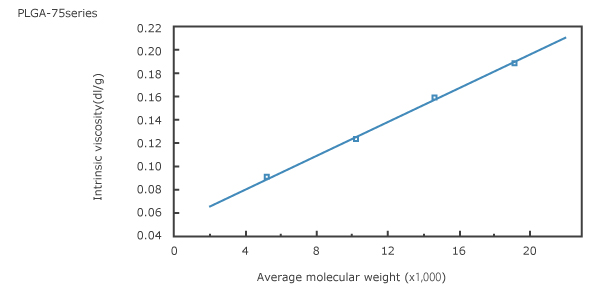
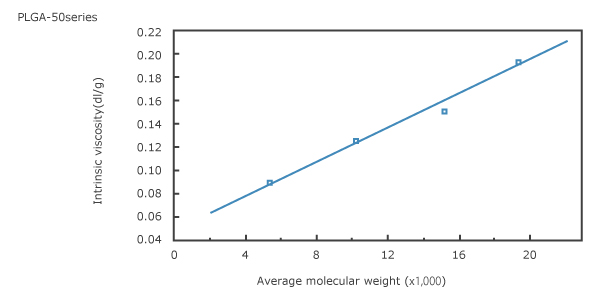
1. Intrinsic viscosity vs. average molecular weight
2. Solubility
| PLA | PLGA | ||||||||||||
| 0005 | 0010 | 0015 | 0020 | 7505 | 7510 | 7515 | 7520 | 5005 | 5010 | 5015 | 5020 | ||
| Methylene chloride | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| Chloroform | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| Acetone | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| Ethyl Acetate | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| THF | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| Toluene | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | △ | △ | △ | △ | |
| Ethanol | △ | △ | △ | △ | △ | △ | △ | △ | △ | △ | × | × | |
| Methanol | △ | △ | △ | △ | △ | △ | △ | △ | △ | △ | × | × | |
| n-Haxane | × | × | × | × | × | × | × | × | × | × | × | × | |
| Water | × | × | × | × | × | × | × | × | × | × | × | × | |
| Test Method | : | solution was prepared by dissolving the polymer in |
| its 25 times by volume of solvent, and solubility | ||
| was expressed by the following criterion |
| Criterion | : | ○ | ; homogeneously soluble |
| △ | ; soluble, but partly remains undissolved | ||
| × | ; very slightly soluble |
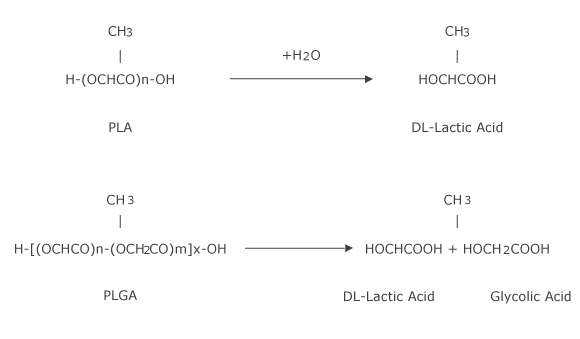
3. Hydrolysis of PLA and PLGA
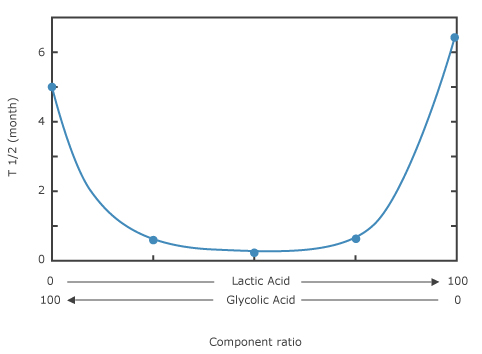
4. Half-life of copolymers with various ratios of Lactic acid and Glycolic acid in implanted rat tissue 1)
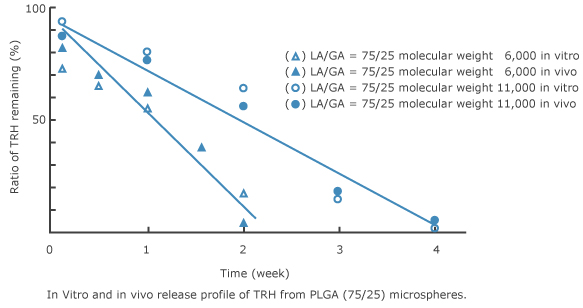
5.Correlation of drug release between in vitro and in vivo 2)
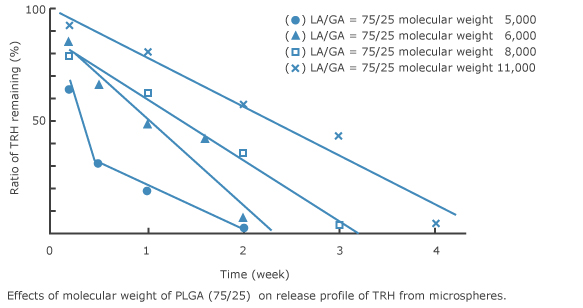
6. Release profile of TRH form PLGA microspheres 2)
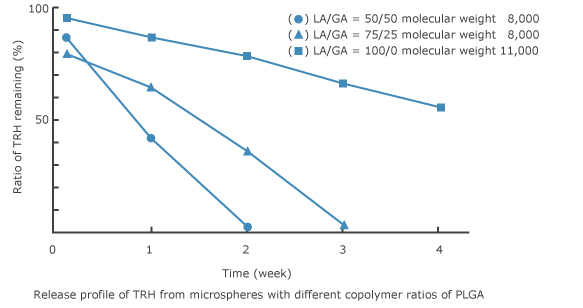
7. Example of preparations
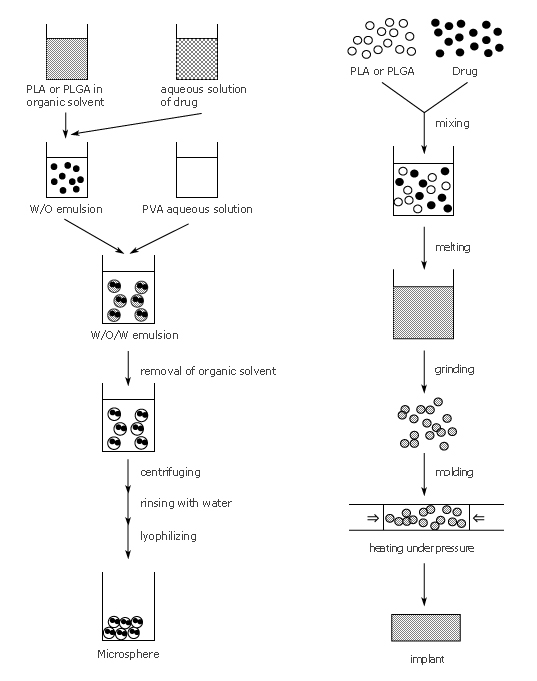
1. Preparation of microsphere 2) 4) 5) In-water drying method is shown |
2. Preparation of implant 3) |
 |
|
References
1) R. Miller, J.M. Brady and D. Cutright, J. Biome. Materd. Res., 11, 711-19 (1977)
2) T. Heye, H. Okada, Y. Ogawa and H. Toguchi, Int. J. Pharm., 72, 199-205 (1991)
3) M. Asano, H. Fukazaki, M. Yoshda, M. Kumakura, T. Mashino, H. Yuasa, K. Imai and H. Yamada, Drug design and delivery,
5, 301-20 (1990)
4) Y. Ogawa, Clinical Pharmacy, 4 (13), 40-43 (1988)
5) Y. Ogawa, M. Yamamoto, H. Okada, T. Yashiki and T. Shimamoto, Chem. Pharm. Bull., 36 (3), 1095-1103 (1988)
Notes